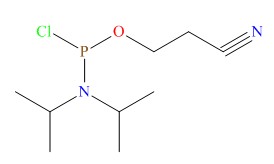
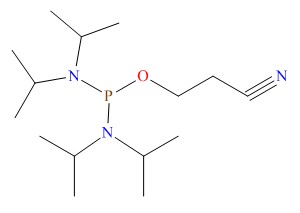
Notice of the General Administration of food and Drug Administration on Issuing 4 guiding principles for the inspection of the quality control of medical devices
Home >> Information >> Detailed content
Product list
Recommended products
news
Contact us
Q Q:27938229
Mobile phone:15981919713
Telephone:0371-56768238
Fax:0371-66658238
Email:chemprize@gmail.com
Mobile phone:15981919713
Telephone:0371-56768238
Fax:0371-66658238
Email:chemprize@gmail.com
Notice of the General Administration of food and Drug Administration on Issuing 4 guiding principles for the inspection of the quality control of medical devices
Time:2016-01-26 15:05:59 Click: author:chemprize
In order to strengthen the supervision and administration of medical device production, on-site inspection and guidance supervision departments in the implementation of the "standard" quality management in the production of medical devices and related appendix to the medical equipment manufacturing enterprises and the inspection results of the assessment, according to the "standard" quality management in the production of medical devices and related appendices, the State Food and drug administration organizations to develop a "medical device production quality management specification for on-site inspection of" guiding principles "medical equipment production quality management standard sterile medical devices on-site inspection of" guiding principles "of medical equipment production quality management standard of implanted medical devices on-site inspection of" guiding principles "medical equipment production quality management standard of in vitro diagnostic reagents, on-site inspection of" guiding principles. Are hereby printed and distributed to you, please follow the instructions.